YOUR CLEANROOM
FOR
MEDICAL DEVICES
Why Cleanrooms Are Essential for High-Quality Medical Device Manufacturing
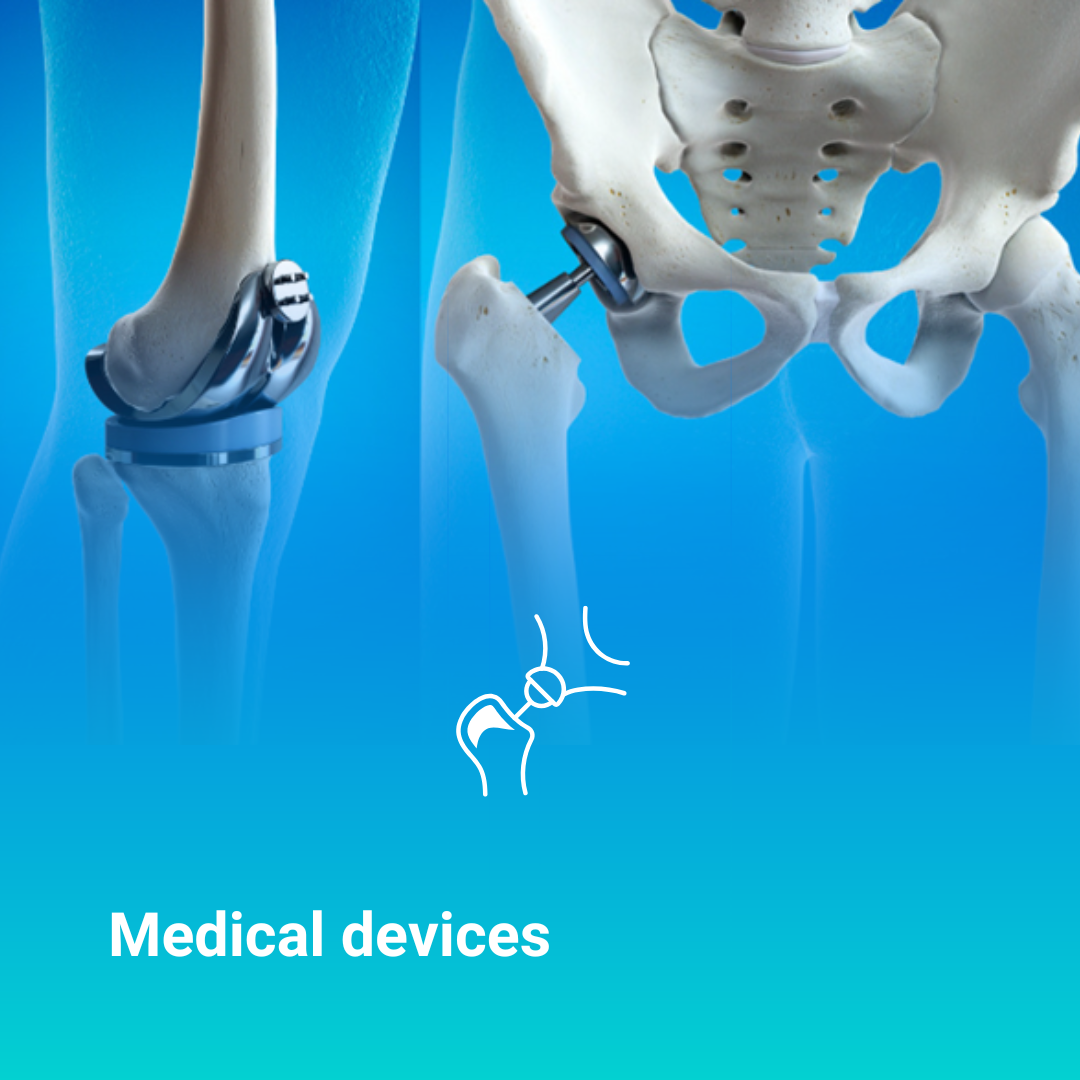
Medical devices are essential products to ensure patient health. Due to their direct contact with the human body, manufacturers are required to produce these devices in ultra-clean, sterile environments. Cleanrooms also known as controlled environments or sterile rooms are essential to minimize microbiological, particulate, and chemical contamination during the manufacturing process.
A medical-grade cleanroom provides a contamination-free, controlled environment that complies with strict regulatory standards for quality, safety, and compliance. This ensures your devices are manufactured under optimal conditions to guarantee patient safety.
Why Cleanrooms Are Essential for High-Quality Medical Device Manufacturing
Medical devices are essential products to ensure patient health. Due to their direct contact with the human body, manufacturers are required to produce these devices in ultra-clean, sterile environments. Cleanrooms also known as controlled environments or sterile rooms are essential to minimize microbiological, particulate, and chemical contamination during the manufacturing process.
A medical-grade cleanroom provides a contamination-free, controlled environment that complies with strict regulatory standards for quality, safety, and compliance. This ensures your devices are manufactured under optimal conditions to guarantee patient safety.
Your Priorities: Reliability, Safety, and Compliance in Cleanroom Production

Control biological and particulate contamination

Minimize the presence of allergenic substances
Ensure patient safety with high-performance devices

Responding to requirements of traceability standards
Your Priorities: Reliability, Safety, and Compliance in Cleanroom Production

Control biological and particulate contamination

Minimize the presence of allergenic substances

Ensure patient safety with high-performance devices

Responding to requirements of traceability standards
Quality, safety and productivity of your sterile room
Whether you’re producing surgical implants, reusable instruments, breast or hip prostheses, or dental equipment, maintaining sterile conditions is non-negotiable. Cleanroom environments are a regulatory necessity to ensure product integrity.
For instance, we designed an ISO 6 cleanroom for one of our clients manufacturing medical fluid bags. The objective was to reduce reject rates by preventing non-compliance during production an investment that significantly improved overall quality and yield.
Customized Cleanroom Solutions for Medical Device Manufacturers
Medical device cleanrooms are closely similar pharmaceutical-grade cleanrooms in terms of performance requirements. After a thorough analysis of your needs and current regulations, we design and build the most technically appropriate solution for your operations.
We consider every critical factor, including :
We also provide expert recommendations on cleanroom equipment such as:
And guidance on:
ottrust us your cleanroom design for Medical Device Manufacturers
Our team helps you define the technical characteristics of your clean zone by analyzing:
Key Cleanroom Equipment We Install:
Euroflux designs and installs fully integrated cleanroom systems tailored to your medical device production needs. Our expertise also extends to the pharmaceutical, cosmetic, and chemical industries.
Our Cleanrooms Also Serve These Industries:
Our Cleanrooms Also Serve These Industries:
Our Cleanrooms Also Serve These Industries: